Gas-phase reactions of NO3 and N2O5 with (Z)-hex-4-en-1-ol, (Z)-hex-3-en-1-ol (‘leaf alcohol’), (E)-hex-3-en-1-ol, (Z)-hex-2-en-1-ol and (E)-hex-2-en-1-ol
Abstract
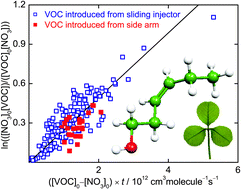
The night-time atmospheric chemistry of the biogenic volatile organic compounds (Z)-hex-4-en-1-ol, (Z)-hex-3-en-1-ol (‘leaf alcohol’), (E)-hex-3-en-1-ol, (Z)-hex-2-en-1-ol and (E)-hex-2-en-1-ol, has been studied at room temperature. Rate coefficients for reactions of the nitrate radical (NO3) with these stress-induced plant emissions were measured using the discharge–flow technique. We employed off-axis continuous-wave cavity-enhanced absorption spectroscopy (CEAS) for the detection of NO3, which enabled us to work in excess of the hexenol compounds over NO3. The rate coefficients determined were (2.93 ± 0.58) × 10−13 cm3 molecule−1 s−1, (2.67 ± 0.42) × 10−13 cm3 molecule−1 s−1, (4.43 ± 0.91) × 10−13 cm3 molecule−1 s−1, (1.56 ± 0.24) × 10−13 cm3 molecule−1 s−1, and (1.30 ± 0.24) × 10−13 cm3 molecule−1 s−1 for (Z)-hex-4-en-1-ol, (Z)-hex-3-en-1-ol, (E)-hex-3-en-1-ol, (Z)-hex-2-en-1-ol and (E)-hex-2-en-1-ol. The rate coefficient for the reaction of NO3 with (Z)-hex-3-en-1-ol agrees with the single published determination of the rate coefficient using a relative method. The other rate coefficients have not been measured before and are compared to estimated values. Relative-rate studies were also performed, but required modification of the standard technique because N2O5 (used as the source of NO3) itself reacts with the hexenols. We used varying excesses of NO2 to determine simultaneously rate coefficients for reactions of NO3 and N2O5 with (E)-hex-3-en-1-ol of (5.2 ± 1.8) × 10−13 cm3 molecule−1 s−1 and (3.1 ± 2.3) × 10−18 cm3 molecule−1 s−1. Our new determinations suggest atmospheric lifetimes with respect to NO3-initiated oxidation of roughly 1–4 h for the hexenols, comparable with lifetimes estimated for the atmospheric degradation by OH and shorter lifetimes than for attack by O3. Recent measurements of [N2O5] suggest that the gas-phase reactions of N2O5 with unsaturated alcohols will not be of importance under usual atmospheric conditions, but they certainly can be in laboratory systems when determining rate coefficients.

 Please wait while we load your content...
Please wait while we load your content...