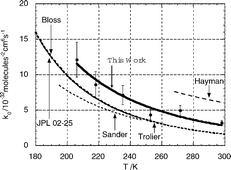
The kinetics of the association reaction of ClO radicals: ClO + ClO + M → Cl2O2
+ M (1), have been investigated as a function of temperature T between 206.0–298.0 K and pressure p between 25–760 Torr using flash photolysis with time-resolved UV absorption spectroscopy. ClO radicals were generated following the photolysis of Br2/Cl2O mixtures in nitrogen diluent gas. Charge coupled device (CCD) detection of time resolved absorptions was used to monitor ClO radicals over a broad wavelength window covering the ClO (A 2Π
← X 2Π) vibronic absorption bands. The high pass filtered ClO absorption cross sections were calibrated as a function of temperature between T
= 206.0–320 K, and exhibit a negative temperature dependence. The ClO association kinetics were found to be more rapid than those reported in previous studies, with limiting low and high pressure rate coefficients, in nitrogen bath gas, k0
=
(2.78 ± 0.82)
× 10−32
×
(T/300)−3.99 ± 0.94 molecule−2 cm6 s−1 and k∞
=
(3.37 ± 1.67)
× 10−12
×
(T/300)−1.49 ± 1.81 molecule−1 cm3 s−1, respectively, (obtained with the broadening factor Fc fixed at 0.6). Errors are 2σ. The pressure dependent ClO association rate coefficients (falloff curves) exhibited some discrepancies at low pressures, with higher than expected rate coefficients on the basis of extrapolation from high pressures (p > 100 Torr). Reanalysis of data excluding kinetic data recorded below p
= 100 Torr gave k0
=
(2.79 ± 0.85)
× 10−32
×
(T/300)−3.78 ± 0.98 molecule−2 cm6 s−1 and k∞
=
(3.44 ± 1.83)
× 10−12
×
(T/300)−1.73 ± 1.91 molecule−1 cm3 s−1. Potential sources of the low pressure discrepancies are discussed. The expression for k0 in air bath gas is k0
=
(2.62 ± 0.80)
× 10−32
×
(T/300)−3.78 ± 0.98 molecule−2 cm6 s−1. These results support upward revision of the ClO association rate coefficient recommended for use in stratospheric models, and the stratospheric implications of the results reported here are briefly discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...