On the statisticodynamical approach of final state distributions in simple bond fissions
Abstract
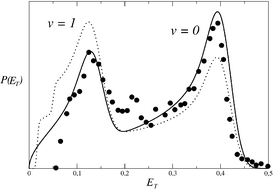
When the energy available to the products of a barrierless unimolecular reaction is of the order of 100 cm−1, phase space theory leads usually to a good description of product state distributions. This is no longer the case for energies of the order of 1000 cm−1. It seems, for example, that the excitation of the recoil energy distribution is underestimated by the theory as suggested by experimental results on the photodissociations of C2O and NCO. As an alternative, we propose a statisticodynamical approach of the previous processes based in part on transition state theory and in part on assumptions relative to the dynamics in the exit-channel. The recoil energy distributions obtained are now in good agreement with the measured ones, in spite of the simplicity of their mathematical expressions.

 Please wait while we load your content...
Please wait while we load your content...