C–H⋯O Hydrogen bonding in 4-phenyl-benzaldehyde: A comprehensive crystallographic, spectroscopic and computational study
Abstract
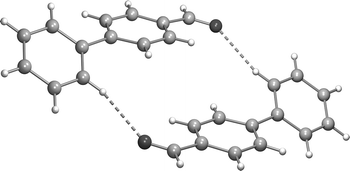
The crystal structure of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C(9)–H groups of adjacent molecules. In the liquid phase, the presence of C–H⋯O bonded forms is revealed by both vibrational and
O and C(9)–H groups of adjacent molecules. In the liquid phase, the presence of C–H⋯O bonded forms is revealed by both vibrational and

 Please wait while we load your content...
Please wait while we load your content...