Superoxide radical anions on the surface of zirconia and sulfated zirconia: formation mechanisms, properties and structure
Abstract
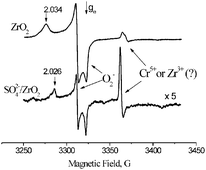
In situ ESR spectroscopy has been used for direct comparison of different thermal and light-induced processes leading to generation of superoxide radical anions on the surface of various zirconia and sulfated zirconia materials. For materials of both types the magnetic resonance parameters of the radical anions were found to be practically independent of the generation method, except for oxygen coadsorption with NO that yields radicals with somewhat smaller gz values. The parameters appear to depend mostly on the state of the surface zirconia cations stabilizing the radical anions, so that the g tensor anisotropy is significantly smaller over sulfated zirconia. It is shown that light-induced formation of superoxide radical anions in the presence of coadsorbed hydrocarbons can be initiated with visible light. Original SIET reaction mechanisms are suggested for the formation of superoxide radical anions by coadsorption with hydrocarbons and illumination after such coadsorption to extend the previously known ones to account for the observed phenomena. Cluster model DFT calculations of magnetic resonance parameters of O2− radical anions stabilized on the surface of zirconium dioxide showed that the adsorption complexes have a T-shape rather than linear structure. The magnetic resonance parameters obtained by calculations practically match experimental data and adequately describe their changes after the surface modification with sulfates.

 Please wait while we load your content...
Please wait while we load your content...