MRD-CI study of the photodissociative behavior of HOOOCl, a molecule relevant to atmospheric chemistry
Abstract
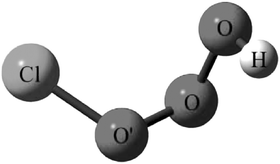
Equilibrium geometries have been optimized and harmonic vibrational frequencies calculated for HOOOCl using quadratic configuration interaction and coupled-cluster methods with Dunning’s correlation-consistent basis sets. There are two conformers of HOOOCl (a cis and a trans structure) that are very close in energy and separated by a barrier of 3.28 kcal mol−1. In experiments, these two forms may co-exist. Rotational constants that can be used to experimentally characterize and identify HOOOCl are presented. The

 Please wait while we load your content...
Please wait while we load your content...