The kinetics of the gas-phase decomposition of the 2-methyl-2-butoxyl and 2-methyl-2-pentoxyl radicals
Abstract
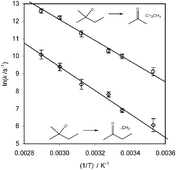
The kinetics of the title reactions have been studied by relative-rate methods as a function of temperature. Relative-rate coefficients for the two decomposition channels of 2-methyl-2-butoxyl have been measured at five different temperatures between 283 and 345 K and the observed temperature dependence is consistent with the results of some previous experimental studies. The kinetics of the two decomposition channels of 2-methyl-2-pentoxyl have also been investigated, as a function of temperature, relative to the estimated rate of isomerisation of this radical. Room-temperature rate coefficient data for the two decomposition channels of both 2-methyl-2-pentoxyl and 2-methyl-2-butxoyl (after combining the relative rate coefficient for this latter with a value for the rate coefficient of the major channel, extrapolated from the data presented by Batt et al., Int. J. Chem. Kinet., 1978, 10, 931) are shown to be consistent with a non-linear kinetic correlation, for alkoxyl radical decomposition rate data, previously presented by this laboratory (Johnson et al., Atmos. Environ., 2004, 38, 1755–1765).

 Please wait while we load your content...
Please wait while we load your content...