Electrochemical reactivity in nanoscale domains: O2 reduction on a fullerene modified gold surface
Abstract
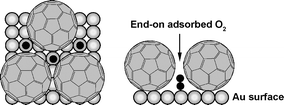
Fullerene is strongly adsorbed on both single crystal and polycrystalline gold surfaces and its specific adsorption resulted in the formation of high coverage large hexagonal rafts with strong interactions between the adsorbed fullerene molecules and the Au substrate. The oxygen reduction reaction (ORR) was investigated on these surfaces to determine their influence on the reduction mechanism. Oxygen reduction did not take place on the fullerene overlayer but proceeded on the sub-nanometer sized exposed pockets of the underlying Au substrate. Reduction at these confined sites produces hydrogen peroxide selectively. This effect is ascribed to the blocking action, or so-called “third body effect”, of the adsorbed fullerene molecules, which do not display electrocatalytic properties for oxygen reduction.

 Please wait while we load your content...
Please wait while we load your content...