Oligomerization and cyclization reactions of acetylene†
Abstract
Acetylene was pyrolyzed in a flow system at pressures between 104 and 403 Torr and temperatures between 854 and 971 K. Products were analyzed by gas chromatography with flame ionization and mass spectrometric detection. In some experiments the reactor was placed in the chromatographic oven to limit condensation. Twenty-four products were detected, of which fifteen were found to be primary. Orders and Arrhenius parameters were found for thirteen products. The results were interpreted in terms of a radical mechanism with 24 reactions for pure acetylene. Radical addition to acetylene produced chemically activated radicals, which could either eliminate a hydrogen atom or add more acetylene. If the radical contained five or more atoms, cyclization to form cyclopentadiene or an aromatic compound, respectively, was also possible. It was suggested that radical recombination could produce chemically activated intermediates that could cyclize to form cyclohexadienes. Addition of neopentane introduced methyl radicals, which could participate in another series of ten chemically activated acetylene addition and hydrogen elimination reactions to form propyne, cyclopentadiene and toluene. Five quotients of rate constants were calculated for competitive reactions.
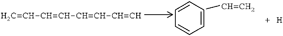

 Please wait while we load your content...
Please wait while we load your content...