Pressure modulation, a new dynamic technique for the electrochemical determination of adsorption, reaction and activation volumes
Abstract
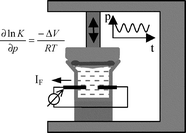
A new dynamic method for the measurement of pressure dependent kinetic and thermodynamic quantities is described and its successful operation demonstrated for two example systems. The pressure was modulated with an amplitude of only ±1 bar or less by means of a piezo-transducer. The small effect on the reaction rate, potential or charge of the electrode can be detected using the lock in technique. The determination of the reaction volume of the redox couple Fe(CN)64−/Fe(CN)63− served as a control of the validity of the measurement and the reliability of the experimental approach. As a first model system the adsorption of hydrogen on polycrystalline Pt was studied. A volume of

 Please wait while we load your content...
Please wait while we load your content...