Crystallisation studies of sodium acetate trihydrate – suppression of incongruent melting and sub-cooling to produce a reliable, high-performance phase-change material†
Abstract
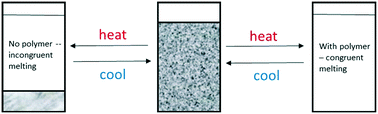
This study has identified two polymer additives that when added in low concentrations can be used to reliably prevent incongruent melting of sodium acetate trihydrate (SAT) when temperature-cycled over multiple thousands of melting and freezing cycles. The polymers appear to act as crystal-habit modifiers that at sub-optimal concentrations dramatically alter the morphology of any anhydrous sodium acetate that may be formed, and at higher concentrations completely suppress precipitation of anhydrous sodium acetate. In situ X-ray powder diffraction experiments show that the active nucleator for SAT is Na2HPO4·2H2O, and that the mechanism of thermally induced deactivation is dehydration in solution to form anhydrous Na2HPO4. Based on these studies, new formulations of SAT have been developed that demonstrate consistent and reliable performance as phase-change materials for heat-storage applications.
- This article is part of the themed collection: Crystal Growth


 Please wait while we load your content...
Please wait while we load your content...