Modelling of an aza-Michael reaction from crystalline naphthalene derivatives containing peri–peri interactions: very long N–C bonds?†
Abstract
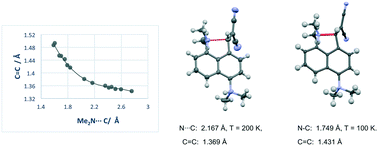
The separation between a pair of peri-located dimethylamino and ethene-2,2-dinitrile groups in a naphthalene molecule, which models the progress of a Michael reaction, can be controlled by the installation of a short ethylene bridge or the introduction of repulsive interactions at the opposite set of peri positions. Introduction of a dimethylammonium substituent produced a hydrated chloride salt in which the Me2N⋯C(H)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CN)2 separation between reactive groups decreases, reversibly, from 2.167 Å at 200 K to 1.749 Å at 100 K, with the maximum rate of change in the range 128–140 K, which was studied by variable temperature X-ray crystallography and solid state NMR. From these and other crystallographic data a correlation between Me2N⋯C bond formation and alkene bond breaking was constructed for the first step of an aza-Michael reaction.
C(CN)2 separation between reactive groups decreases, reversibly, from 2.167 Å at 200 K to 1.749 Å at 100 K, with the maximum rate of change in the range 128–140 K, which was studied by variable temperature X-ray crystallography and solid state NMR. From these and other crystallographic data a correlation between Me2N⋯C bond formation and alkene bond breaking was constructed for the first step of an aza-Michael reaction.


 Please wait while we load your content...
Please wait while we load your content...