Octanuclear nickel phosphonate core forming extended and molecular structures†
Abstract
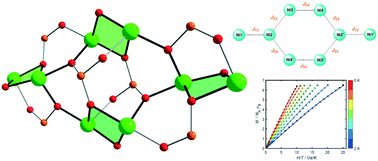
Three new nickel phosphonate complexes {[Na2Ni8(L)6]·nSolv}m (L = SAA3− (1), BSAA3− (2), NAA3− (3); Solv = H2O, MeOH; m = ∞ (1, 2), 1 (3)) were synthesized. All three complexes possess a novel octanuclear {Ni8} phosphonate core, which consists of four dinuclear doubly oxygen-bridged units, further interconnected to each other by phosphonate oxygen bridges. The steric features of the ligands influence the aggregation degree. Molecules of 1 and 2 are interconnected by sodium cations into 2D layered and 1D chain extended structures, respectively, while the molecules of 3 with the bulkiest ligand are not bonded with each other. Magnetic properties of the obtained {Ni8} core unit were studied for complex 1 as a representative of this family of compounds and are reported in detail. Magnetic susceptibility at low temperature is indicative of a singlet ground state. The absence of saturation and the magnetization behavior points to zero-field splitting (ZFS). Simulation of the magnetization data revealed an easy-plane magnetic anisotropy with an axial ZFS parameter D = 7.4 cm−1. The magnetic properties of 1 were also studied by broken-symmetry DFT calculations (BS-DFT), which revealed the presence of ferromagnetic exchange interactions within the dinuclear units of the {Ni8} core with a dominant antiferromagnetic interaction between these dinuclear entities. These results are in good agreement with coupling constants derived from the experimental susceptibility and magnetization data.


 Please wait while we load your content...
Please wait while we load your content...