Semi-conductive helical homochiral metal–organic frameworks based on enantiomeric proline derivatives†
Abstract
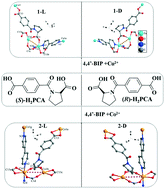
Among various chiral compounds, amide derivatives modified from amino acids are attractive chiral synthons in the synthesis of homochiral metal–organic frameworks. Herein, a pair of proline derivatives, (S)-1-(4-carboxybenzoyl) pyrrolidine-2-carboxylic acid ((S)-H2PCA) and (R)-1-(4-carboxybenzoyl) pyrrolidine-2-carboxylic acid ((R)-H2PCA), were designed and synthesized through incorporating a proline unit into 1,4-dicarboxybenzene. Under solvothermal conditions, (S)-H2PCA and (R)-H2PCA further reacted with 4,4′-bipyridine (4,4′-BIP), Cu(II) and Co(II) ions to obtain four enantiomeric compounds, [Cu((S)-PCA)(4,4′-BIP)]·DEF·H2O(1-L), [Cu((R)-PCA)(4,4′-BIP)]·DEF·H2O(1-D), [Co((S)-PCA)(4,4′-BIP)]·1.5H2O (2-L) and [Co((R)-PCA)(4,4′-BIP)]·1.5H2O (2-D) (DEF = N,N-diethyl formamide). Although all compounds have pillared-layer frameworks with 6-connected pcu nets, their structural motifs are still very different. The frameworks of 1-L and 1-D are non-interpenetration structures containing two kinds of helical chains, while 2-L and 2-D are 2-fold interpenetration frameworks with only one kind of helical chain. Furthermore, their solid-state CD spectra, semi-conductive properties and photocatalytic behaviors were also investigated. The present work provides a promising method to design and synthesize homochiral MOFs with helical structures through semirigid (S)-H2PCA and (R)-H2PCA.


 Please wait while we load your content...
Please wait while we load your content...