Supramolecular lead(ii) architectures engineered by tetrel bonds†
Abstract
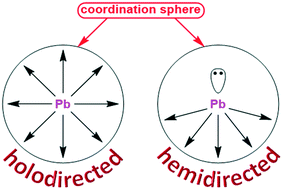
In this work, we have directed our attention to the evaluation of closely related bis-pyridyl ligands N′-(pyridin-2-ylmethylene)picolinohydrazide (HLI), N′-(pyridin-2-ylmethylene)nicotinohydrazide (HLII) and N′-(1-(pyridin-2-yl)ethylidene)isonicotinohydrazide (HLIII) as linkers for PbII extended structures. The reaction of Pb(NO3)2 or Pb(ClO4)2 with an equimolar amount of HLI–III in the presence of KSCN in EtOH at 60 °C leads to the formation of heteroleptic coordination polymers [Pb(HLI)(NO3)2]n, [Pb(LII)(ClO4)]n and [Pb(HLIII)(NO3)(NCS)]n. The structures of all these complexes are highly dictated by both the structure of the parent organic ligand and the nature of inorganic anions. In all cases, the main structural motif is a zig-zag 1D coordination polymer, which can be further extended to higher dimensions due to tetrel bonds, N–H⋯O hydrogen bonds and π⋯π interactions. The formation of tetrel bonds was possible thanks to a hemidirected coordination sphere of the PbII atoms in all the described complexes. As a result, the overall coordination number of the metal center was found to be 10, 7 and 8 in [Pb(HLI)(NO3)2]n, [Pb(LII)(ClO4)]n and [Pb(HLIII)(NO3)(NCS)]n, respectively. DFT calculations were performed in order to determine the nature of non-covalent interactions and stability of the obtained structures.


 Please wait while we load your content...
Please wait while we load your content...