Two new MOFs based on 5-((4-carboxypyridin-2-yl)oxy) isophthalic acid displaying unique selective CO2 gas adsorption and magnetic properties†
Abstract
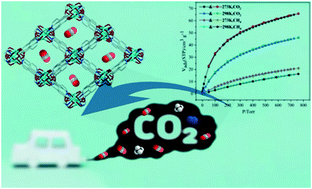
Herein, two new water-stable magnetic frameworks, [Cu1.5(L)(H2O)]·2H2O (1) and [Co4(L)2(CH3CN)(OH)2(H2O)4]·3H2O (2) (H3L = 5-((4-carboxypyridin-2-yl)oxy) isophthalic acid), were synthesized by a solvothermal method in similar reaction systems and characterized by infrared spectroscopy, powder X-ray diffraction and thermogravimetric measurements. Due to their different metal ions, 1 shows a trinodal (4,4,4)-connected three-dimensional (3D) framework that possesses 1D channels based on [Cu2(COO)4] paddle-wheel secondary building units (SBUs), whereas 2 is a 2D layered network based on tetranuclear [Co4(COO)6(μ3-OH)2] SBUs and reveals a (3,6)-connected kgd topological structure. The gas adsorption explorations demonstrated that 1 exhibited commendable selectivity for CO2 over CH4. Moreover, both 1 and 2 showed different degrees of antiferromagnetic exchange action.


 Please wait while we load your content...
Please wait while we load your content...