Chloride binding capacity of LDHs with various divalent cations and divalent to trivalent cation ratios in different solutions
Abstract
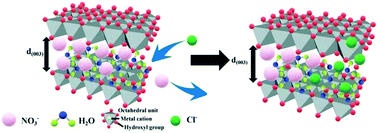
Layered double hydroxides (LDHs) have shown great potential to prevent chloride penetration into cementitious materials. This paper intends to investigate the effect of the divalent cation type and divalent to trivalent metal ion ratio of LDHs on their chloride binding capacity. Different types of M2+ (Mg, Ca, Zn)–Al–NO3 LDHs were prepared by a hydrothermal synthesis method. Scanning electron microscopy (SEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR) and thermogravimetry and differential scanning calorimetry (TG-DSC) were adopted to characterize the synthesized LDHs. In addition, the chloride binding capacity of LDHs was measured using the equilibrium isotherm of chloride binding. These results indicated that Zn–Al–NO3 LDH (Z-LDH) exhibited the largest chloride binding capacity because of its greatest basal spacing compared with Mg–Al–NO3 LDH (M-LDH) and Ca–Al–NO3 LDH (C-LDH). The chloride binding capacity of Z-LDH increased with the decrement of the divalent to trivalent metal ion ratio ranging from 2 to 4. Due to the competitive binding of anions, the chloride binding capacity of LDHs in simulated concrete pore (SCP) solution was slightly lower than that in deionized water. However, simulated carbonation of the SCP solution resulted in considerable desorption of chloride. Special attention should be paid to the desorption of chlorides caused by carbonation if LDHs are used in cementitious materials to prevent chloride penetration.


 Please wait while we load your content...
Please wait while we load your content...