Hydrochromism behaviors of solid forms of chelerythrine hydrochloride†
Abstract
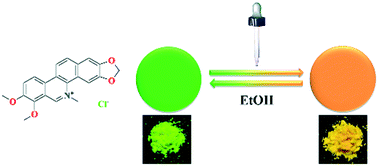
Chelerythrine hydrochloride (CHE-Cl), a salt of an unstable naturally fluorescent material, exhibited reversible hydrochromism fluorescence emissions. Three solid forms with high crystallinity were obtained via comprehensive crystallization investigations. Two of them presented green emission (G1: λem = 509.8 nm; G2, λem = 522.0 nm), and the other showed orange emission (O, λem = 558.8 nm) under 365 nm UV irradiation. Reversible water-triggered changes in the fluorescence color of CHE-Cl between G1/G2 and O were observed and fully characterized. Solid-state fluorescence spectroscopy (SFS), X-ray powder diffraction (XRPD) and single-crystal X-ray diffraction (SCXRD) experiments indicated that conformational changes and water-induced rearrangement of the crystal lattice under different stimuli were responsible for the reversible switching of the fluorescence between the solid forms of CHE-Cl. Density functional theory (DFT) calculations revealed that HOMO–LUMO energy band gaps were highly affected by the conformations and packing patterns of the CHE-Cl molecules. This result suggested an alternative approach involving salt formation to develop stimuli–response luminescent materials, especially for those with unstable chemical structures in the free base form.


 Please wait while we load your content...
Please wait while we load your content...