Molecular tectonics: enantiomerically pure chiral crystals based on trans-1,2-cyclohexanediol†
Abstract
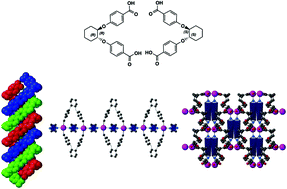
Enantiomerically pure chiral trans-1,2-cyclohexanediol ((R,R) or (S,S)) based organic compounds 3b and c bearing two benzoic acid moieties are self-complementary units and thus self-assemble in the crystalline phase into isostructural crystals composed of 1D helical H-bonded networks with opposite handedness. The helical strands form enantiomerically pure triple helices. The racemic mixture of the two chiral ligands 3a also self-assembles into 1D achiral H-bonded networks displaying a zigzag type geometry. Upon deprotonation, both chiral 3b and 3c bearing two carboxylate moieties behave as coordinating ligands. When combined with Cd(NO3)2 in the presence of ancillary N donor ligands such as Dabco (1,4-diazabicyclo[2.2.2]octane) or Bipy (4,4′-bipyridine)), they lead to the formation of enantiomerically pure coordination networks. Whereas both Cd-3b-Dabco and Cd-3c-Dabco 1D networks, resulting from the interconnection of 2–2 metallamacrocycles by Dabco behaving as a linear connector, are observed, in the case of the 4,4′-Bipy connector (Cd-3b-Bipy and Cd-3c-Bipy), a 2D coordination network based on the interconnection of binuclear Cd(II) dimers by coordinating ligands and ancillary ligands is generated.
- This article is part of the themed collection: Introducing the CrystEngComm Advisory Board and their research


 Please wait while we load your content...
Please wait while we load your content...