Structural design of alkali-metal titanates: electrochemical growth of KxTi8O16, Na2+xTi6O13, and Li2+xTi3O7 single crystals with one-dimensional tunnel structures†
Abstract
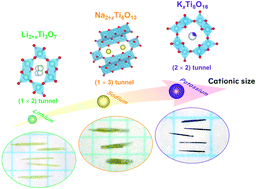
Single crystals of lithium, sodium, and potassium titanates were grown with high-temperature constant-voltage electrolysis of TiO2 with A2MoO4 (A = Li, Na, and K) as fluxes. Using the potassium-containing flux, needle-like crystals of hollandite-type KxTi8O16 with a maximum length of ∼1 mm were successfully obtained. On the other hand, needle-like crystals of Na2+xTi6O13 and ramsdellite-type Li2+xTi3O7 with a maximum length of ∼3 mm were grown when the sodium- and lithium-containing fluxes were employed, respectively. This report demonstrates that the electrochemical crystal growth is favorable to obtain titanates with various one-dimensional tunnel structures upon varying the alkali-metal elements. The diversity in structural types of the resultant single crystals is reasonably explained in terms of the ionic size of the alkali metals incorporated.


 Please wait while we load your content...
Please wait while we load your content...