Ln(iii)-Containing polyoxomolybdates based on β-{Mo8O28}: microwave synthesis and optical and magnetic properties†
Abstract
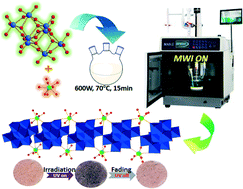
Two isomorphic compounds {[Ln2(H2O)10](Mo8O27)}·8H2O (Ln = Tm (1), Er (2)) were obtained by microwave irradiation, in which β-{Mo8O28} building units are linked via bridge oxygen and lanthanide ions coordinated with {MoO6} octahedra. The synthesis chemical processes have a short reaction time (15 min) with high yields (exceeding 85%). The solid-state photochromic properties of these two compounds were studied, and their color change process and the photochromic mechanism were analyzed. Furthermore, the solid-state photoluminescence properties of these two compounds were investigated and the stability of the luminescence was verified. Finally, variable temperature magnetic susceptibilities indicate that both compounds have anti-ferromagnetic interaction properties and 1/χM values are linear with temperature.


 Please wait while we load your content...
Please wait while we load your content...