Binaphthanol-based organic fluorophores with color tunability and their optical properties†
Abstract
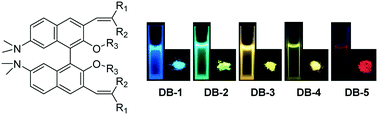
Luminescent organic materials with tunable emission color have attracted much attention for their potential application in multiple fields. Herein, we designed and synthesized a series of dimethylamine substituted binaphthanol based organic fluorophores. Decorating with substituents at the alkene moiety on the binaphthanol core backbone led to organic solid emitters with color tunability from the blue to red regions. These fluorophores exhibited solvatochromic properties and two compounds (DB-4 and DB-5) showed aggregation-induced emission properties. The binaphthanol core structure induced a twisted molecular conformation and special intermolecular packing mode in a single crystal structure, which was beneficial for the inhibition of the strong intermolecular π–π stacking interaction and induced the AIE feature as well as the fluorescence emission in the solid state. The absorption and emission changes of these molecules under acidic and basic conditions revealed that they had acidochromic properties and could be used as security ink. This work provided a new strategy for the synthesis of organic solid emitters with tunable emissions based on the simple binaphthanol core backbone.


 Please wait while we load your content...
Please wait while we load your content...