Rational synthesis of silver nanowires at an electrode interface by diffusion limitation
Abstract
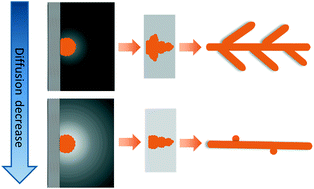
We report an approach to synthesize silver nanowires by diffusion limitation. Silver particles are synthesized by electrochemical reaction in mixtures of silver nitrate solution and glycerol. Without glycerol, the main product is silver dendrites. With the addition of glycerol, silver nanowires are instead produced in abundance. The addition of glycerol increases the viscosity of the solvent, which restricts the diffusion of silver ions. Under conditions of diffusion limitation, a rapid electrochemical reaction leads to the formation of a chemical concentration gradient at the growth front of the nuclei, which results in the formation of silver dendritic structures. The increase of solvent viscosity attenuates the concentration gradient, which limits secondary nucleation on the sides of the silver rods, leading to the formation of 1D silver nanowires instead of dendrites. An increase of silver salt concentration sharpens the concentration gradient, which leads to the formation of dendritic structures again, confirming the dominant role of the interface chemical distribution in the structural evolution of the product materials. The silver nanowires synthesized are used to fabricate a conductive film by vacuum filtration followed by fixing and welding processes. The film shows high conductivity and excellent flexibility.


 Please wait while we load your content...
Please wait while we load your content...