Crystal nucleation from solutions of proteins with temperature-independent solubility: a case study of apoferritin
Abstract
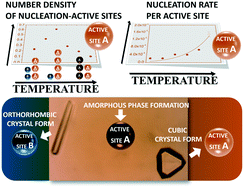
Apoferritin is a thermostable protein cage that belongs to those proteins whose solubility does not exhibit dependence on temperature. This opens up a possibility for investigation of its crystal nucleation over a large temperature interval with the aim of focusing on possible kinetic effects on the process. We studied the temperature dependence of apoferritin nucleation in the temperature range of 10–60 °C in a batch crystallization setup. The probability for detection of at least one crystallite per solution volume until a given time and at a given temperature was determined. We found that the nucleation of cubic apoferritin crystals occurs on nucleation-active sites and the nucleation rate per active site was obtained. The temperature-dependent values of the work for nucleus formation, the number of apoferritin molecules in the nucleus, the frequency of attachment of molecules to the nucleus, the Zeldovich factor and the nucleation time lag under the experimental conditions were determined. Two hypotheses for the variation of the average number density of the nucleation sites with temperature were suggested: (i) high temperatures lead to a competition for active sites between the crystallization of the cubic crystal form and amorphous phase formation, and (ii) low temperatures facilitate the formation of a second type of nucleation-active sites which lead to slow nucleation of the orthorhombic apoferritin crystal form. Our study reveals that the temperature could significantly affect apoferritin crystal nucleation, apart from its common role as a strong determinant of the supersaturation in most of the crystallization systems.


 Please wait while we load your content...
Please wait while we load your content...