Nickel phosphide polymorphs with an active (001) surface as excellent catalysts for water splitting†
Abstract
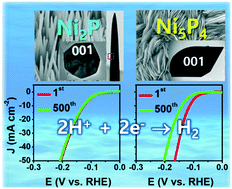
Since the emergence of hydrogen generation by water-splitting as a core renewable-energy technology, the development of related catalysts with high efficiency, long-term stability, and low cost has been vigorously pursued. We report the temperature-controlled synthesis of two nickel phosphide polymorphs, Ni2P and Ni5P4, by phosphorization of Ni foil or foam using phosphine gas. The hexagonal phase Ni2P nanowires and Ni5P4 nanosheets were grown on Ni substrates with vertical alignment, and uniformly exposed active (001) planes. The Ni5P4 nanosheets possess significant stacking faults along the [0001] direction. Both Ni2P and Ni5P4 exhibit excellent electrocatalytic activity toward the hydrogen evolution reaction (HER). Their overpotential for 10 mA cm−2 was 0.126 and 0.114 V, and the Tafel slope was 42 and 34 mV dec−1 in 0.5 M H2SO4 electrolyte, respectively. A decrease in HER performance was observed for Ni5P4, but the change was negligible for Ni2P. Strain mapping using a precession-assisted nanobeam electron diffraction technique showed that only Ni5P4 underwent degradation of basal (001) planes during HER, which explains the lower stability of catalytic activity. Furthermore, the Ni2P nanowires demonstrated excellent catalytic activity toward overall water splitting, which could be attributed to the stable surface as well as the highly conductive crystal structures.


 Please wait while we load your content...
Please wait while we load your content...