High index facet bounded α-Fe2O3 pseudocubic nanocrystals with enhanced electrochemical properties: Zn2+ ion assisted solvo-hydrothermal synthesis
Abstract
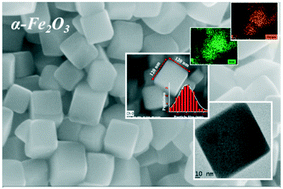
Pseudocubic α-Fe2O3 nanocrystals were grown by a surfactant-free, low temperature, solvo-hydrothermal process and characterised by XRD, FESEM, TEM, FTIR, Raman, XPS and UV-vis analysis. These α-Fe2O3 nanocrystals were bounded by high index facets {012}, {01![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) } and {
} and {![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 10} having an average edge length of 120 nm and a dihedral angle of 85°. The growth of high index facet bounded α-Fe2O3 pseudocubic nanocrystals was contributed by the structure inducing properties of Zn2+ cations (ZnCl2) added to the mother solution. This work also sheds light into the low impedance and enhanced electrochemical activities of high index facet bounded α-Fe2O3 pseudocubic nanocrystals, which exhibited good electrochemical sensing response towards aqueous ammonia.
10} having an average edge length of 120 nm and a dihedral angle of 85°. The growth of high index facet bounded α-Fe2O3 pseudocubic nanocrystals was contributed by the structure inducing properties of Zn2+ cations (ZnCl2) added to the mother solution. This work also sheds light into the low impedance and enhanced electrochemical activities of high index facet bounded α-Fe2O3 pseudocubic nanocrystals, which exhibited good electrochemical sensing response towards aqueous ammonia.


 Please wait while we load your content...
Please wait while we load your content...