Unipolar to ambipolar semiconductivity switching in charge transfer cocrystals of 2,7-di-tert-butylpyrene†
Abstract
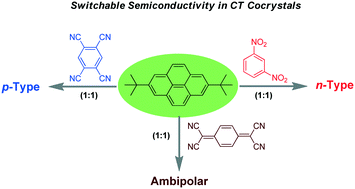
Incorporating the concept of heteroatom doping induced charge polarity switching in inorganic semiconductors, we report the switching of ambipolar to unipolar p/n-type semiconductivity by the charge transfer cocrystallization of a π-donor 2,7-di-tert-butylpyrene (di-t-BuPy) with a π-acceptor 7,7′,8,8′-tetracyanoquinodimethane (TCNQ), tetracyanobenzene (TCNB), or 1,3-dinitrobenzene (1,3-DNB). The charge transfer cocrystals were characterized by single crystal XRD, IR, EPR and UV-Vis spectroscopy in the solid state. The origin of the charge polarity switching was elucidated by the super-exchange pathway, which emphasizes the importance of the bridge orbital for the charge carrier transport in addition to the transport orbitals. The switching of the semiconductor nature from ambipolar to unipolar stems from the definitive role of bridge orbitals in charge transport. Subsequently, the switching of semi-conductivity was verified by experimental studies on organic field effect transistor (OFET) type devices.


 Please wait while we load your content...
Please wait while we load your content...