Unique crystallographic signatures of Boc-Gly-Phe-Phe-OMe and Boc-Gly-Phg-Phe-OMe and their self-association†
Abstract
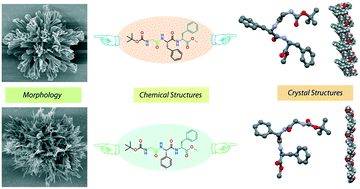
The self-assembly of N- and C-protected tripeptides, Boc-Gly-Phe-Phe-OMe (1) and its analog Boc-Gly-Phg-Phe-OMe (2, Phg = phenylglycine), has been investigated. The presence of just an extra methylene (–CH2–) group in the side chain of one of the amino acids resulted in significant changes in their molecular arrangement and supramolecular structure. The single crystal X-ray diffraction analysis suggested that 1 adopted a type II β-turn-like conformation, known as open turn identified by the absence of any intramolecular hydrogen bond, which further self-assembled to form a herringbone helix-like architecture through non-covalent interactions. To the best of our knowledge, this is the first report on a designed open turn tripeptide without a kink-forming element. However, in spite of the presence of a non-standard amino acid 2 adopted a β-sheet conformation which further self-organized to form a helical architecture through non-covalent interactions in the crystalline form. The conformations of these peptides in solution were also investigated by solvent dependent NMR titration, 2D NOESY, and CD spectroscopic experiments. These peptides exhibited two different flower-like architectures in acetonitrile–water medium under an optical microscope and a field emission scanning electron microscope (FESEM).


 Please wait while we load your content...
Please wait while we load your content...