Synthesis of hydrous cobalt phosphate electro-catalysts by a facile hydrothermal method for enhanced oxygen evolution reaction: effect of urea variation
Abstract
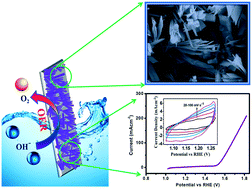
In this report, highly active hydrous cobalt phosphate thin film electro-catalysts were synthesized by a low cost and one-pot hydrothermal method at 393 K and were evaluated for the oxygen evolution reaction (OER). The impact of varying the hydrolyzing agent, urea, on the structural and morphological properties of hydrous cobalt phosphate thin films and the consequent electro-catalytic performances was studied. The morphology of hydrous cobalt phosphate thin films resembles a microflower-like structure consisting of microplates. Upon urea variation, the microplates undergo changes in thickness and width and eventually transform into microflakes. The remarkable electrocatalytic performance of hydrous cobalt phosphate thin films was observed towards the OER and they exhibit a low overpotential of 292 mV (vs. RHE) at 10 mA cm−2 current density with a Tafel slope of 98 mV dec−1 in 1.0 M KOH electrolyte. Also, the stability of the electrocatalyst is demonstrated by continued electrolysis, showing that the hydrous cobalt phosphate thin film electro-catalyst sustains superior long-term electrochemical stability for 10 h at 10 mA cm−2 constant current density. The enhanced electrocatalytic performance of hydrous cobalt phosphate thin film electro-catalysts was attributed to a high ECSA (1210 cm−2) and low electrochemical impedance imparted by the microflower structure with microflake-like morphology.


 Please wait while we load your content...
Please wait while we load your content...