3-Cyanopyridine as a bridging and terminal ligand in coordination polymers†
Abstract
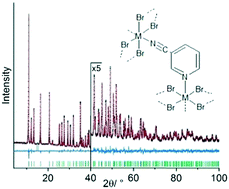
3-Cyanopyridine (3-CNpy) can act as a bridging or terminal ligand in transition metal coordination polymers. The compounds [MIIBr2(3-CNpy)4] with M = Mn, Fe, Co, Ni (1a–4a) consist of individual complexes, in which the metal atom is octahedrally coordinated by two bromine atoms and four 3-cyanopyridine ligands, which coordinate through their pyridine N atoms only. Thermal decomposition at around 160 °C leads to the release of two 3-cyanopyridine molecules and the formation of [MIIBr2(3-CNpy)2]n with M = Mn, Fe, Co, Ni (1b–4b). For 3b and 4b two polymorphs were observed each (α-3b, β-3b, α-4b, β-4b). In all six phases the metal atoms are linked by bromine bridges into [MIIBr2]n chains. The 3-cyanopyridine ligands again act as terminal ligands coordinating with their pyridine N atoms. Upon further heating to around 250 °C, the compounds [MIIBr2(3-CNpy)1]n with M = Mn, Fe, Co, Ni (1c–4c) are obtained. 1c–4c are again built up from [MIIBr2]n chains with lateral 3-CNpy ligands, but additionally the cyano groups coordinate to the metal atoms. 1c–4c are the first compounds with 3-cyanopyridine as a bridging ligand between two 3d M2+ ions. The bridges connect neighbouring [MIIBr2]n chains and this results in the formation of a wavy, two-dimensional network. All crystal structures were determined from X-ray powder diffraction data.


 Please wait while we load your content...
Please wait while we load your content...