A new europium metal–organic framework with both high proton conductivity and highly sensitive detection of ascorbic acid†
Abstract
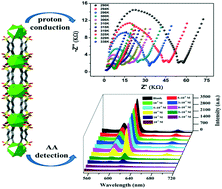
A new metal–organic framework (MOF), [{(H3O)[Eu (SBDB)(H2O)2]}n] (H4SBDB = 1,5-disulfo-benzene-2,4-dicarboxylic acid) (1), was successfully synthesized under solvothermal conditions. Ten central Eu3+ ions are coordinated by the H4SBDB ligands and two coordination water molecules. The adjacent Eu3+ ions are connected by oxygen atoms from carboxyl groups, forming a 1D chain. 1D chains are linked by H4SBDB ligands to induce a 2D infinitely extensible planar structure, and then connected by hydrogen bonds to form a 3D supernetwork structure. Complex 1 exhibits excellent thermal and chemical stability. AC impedance analysis shows that the highest proton conductivity of 1 reaches up to 1.0 × 10−4 S cm−1 (338 K, 98% RH). Furthermore, complex 1 is an excellent luminescence-based sensor with high sensitivity and a low detection limit for ascorbic acid (AA) in aqueous solutions.


 Please wait while we load your content...
Please wait while we load your content...