Effect of rGO–Fe2O3 nanocomposites fabricated in different solvents on the thermal decomposition properties of ammonium perchlorate
Abstract
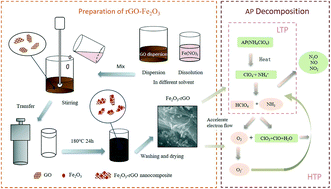
rGO–Fe2O3 composites were prepared using different solvents (distilled water, ethanol, N-methylpyrrolidone, ethyl acetate, n-butyl alcohol and N,N-dimethylformamide) via a simple solvothermal method and characterized by SEM, TEM, XRD, FT-IR, Raman and XPS techniques. Then, the catalytic action of rGO–Fe2O3 composites (prepared in different solvents), GO, rGO and pristine Fe2O3 on the thermal decomposition of ammonium perchlorate (AP) were studied using a TG-DSC instrument. DSC results showed that GO, rGO, pristine Fe2O3 and rGO–Fe2O3 composites could effectively promote the thermal decomposition of AP. Additionally, rGO–Fe2O3 fabricated in N,N-dimethylformamide (DMF) solvent possesses the best catalytic performance among the investigated materials. This result can be attributed to the better dispersibility of Fe2O3 nanoparticles on graphene oxide prepared in DMF solvent, which was confirmed by SEM and TEM imaging. The high temperature decomposition exothermic peak (HTP) and the apparent activation energy of AP are reduced by 119.6 °C and 173.3 kJ mol−1, respectively, in the presence of Fe2O3–rGO (DMF) composite.


 Please wait while we load your content...
Please wait while we load your content...