Insights on conformation in the solid state: a case study – s-cis and/or s-trans crystallization of 5(3)-aryl-3(5)-carboxyethyl-1-tert-butylpyrazoles†
Abstract
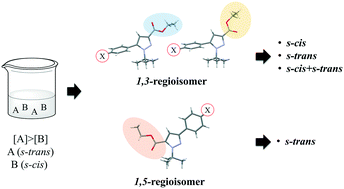
A series of 5(3)-aryl-3(5)-carboxyethyl-1-tert-butylpyrazoles (aryl, 4-X-C6H4, where X = H, F, Cl and Br) were studied in the solid state. The 1,3-regioisomers (carboxyethyl group in 3-position) of t-butylpyrazoles crystalized in three different forms: s-cis, s-trans or s-cis + s-trans. On the other hand, the 1,5-regioisomers (carboxyethyl group in 5-position) of t-butylpyrazoles showed only s-trans conformation. The 13C CPMAS and SCXRD data confirmed that each 1,3- and 1,5-regioisomers of t-butylpyrazoles crystallized only one of the three mentioned forms. The potential energy surface (PES) yielded insights regarding the formation of each conformer. In general, quantum mechanical calculations showed that the conformer s-trans is more stable than s-cis, and the calculated stability difference was 0.7 kcal mol−1 for the 1,3-regioisomers and 3.5 kcal mol−1 for the 1,5-regioisomers. Moreover, 1,5-regioisomers of t-butylpyrazoles showed intramolecular interactions of type CH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C between the carbonyl and t-butyl group, which was obtained by QTAIM analysis. This interaction can influence the stabilization for s-trans conformation in the solid state. In contrast, the 1,3-regioisomers did not show intramolecular interaction with the COOEt group, and the conformation adopted in the solid state should be the consequence of the crystalline packing. The QTAIM analysis of the more stable dimers of s-trans-conformation for X = Cl, Br showed that the halogen atoms interact with the COOEt group, helping stabilize this conformation. On the other hand, in the s-cis⋯s-cis conformation dimer (X = Cl, Br), the COOEt group was stabilized by the phenyl group, which is the same stabilization for X = H.
C between the carbonyl and t-butyl group, which was obtained by QTAIM analysis. This interaction can influence the stabilization for s-trans conformation in the solid state. In contrast, the 1,3-regioisomers did not show intramolecular interaction with the COOEt group, and the conformation adopted in the solid state should be the consequence of the crystalline packing. The QTAIM analysis of the more stable dimers of s-trans-conformation for X = Cl, Br showed that the halogen atoms interact with the COOEt group, helping stabilize this conformation. On the other hand, in the s-cis⋯s-cis conformation dimer (X = Cl, Br), the COOEt group was stabilized by the phenyl group, which is the same stabilization for X = H.


 Please wait while we load your content...
Please wait while we load your content...