A facile route to the controlled synthesis of β-NaLuF4:Ln3+ (Ln = Eu, Tb, Dy, Sm, Tm, Ho) phosphors and their tunable luminescence properties†
Abstract
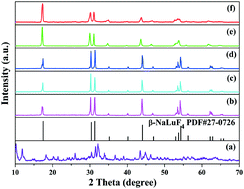
Highly uniform and monodisperse β-NaLuF4:Ln3+ (Ln = Eu, Tb, Dy, Sm, Tm, Ho) hexagonal prisms have been synthesized via a facile two-step hydrothermal method without any organic surfactants. The structures, morphologies and luminescence properties of the samples were investigated through XRD, SEM, and PL spectra. Furthermore, the shapes and sizes of the products can be further manipulated through adjusting the pH values of initial solutions. Upon excitation at 368 nm, the β-NaLuF4:Tb3+ phosphors exhibit the strongest green emission at pH = 9.0. Moreover, the photoluminescence properties of β-NaLuF4:Ln3+ were studied in detail, and it was found that the photoluminescence color of the β-NaLuF4:0.03Tm3+ phosphor was close to standard blue light (0.14, 0.08). Under 356 nm irradiation, multicolour emission from blue to sapphire and then to pale green has been achieved from the Tm3+/Dy3+ co-doped β-NaLuF4 samples. The energy transfer process from Tm3+ to Dy3+ was studied and was demonstrated to be an electric dipole–dipole interaction mechanism. It is obvious that the β-NaLuF4 phosphors may have potential applications in the field of full-color displays.


 Please wait while we load your content...
Please wait while we load your content...