Oxyanion clusters with antielectrostatic hydrogen bonding (AEHB) in tetraalkylammonium hypodiphosphates†
Abstract
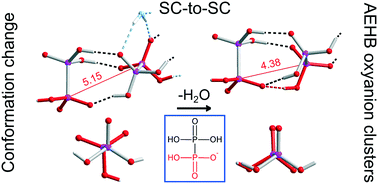
Organic–inorganic salts of hypodiphosphoric acid with tetraalkylammonium cations have been synthesized and characterized by X-ray crystallography and IR spectroscopy. [(Et4N)(H3P2O6)]·0.5H4P2O6·2H2O (1), [(Et4N)2(H2P2O6)]·H4P2O6 (2), [(n-Bu4N)(H3P2O6)]·0.75H4P2O6 (3), [(n-Bu4N)(H3P2O6)]·0.25H4P2O6·0.53H2O (4), [(n-Bu4N)(H3P2O6)]·0.25H4P2O6 (5) and [(Et4N)Cl]·0.5H4P2O6·3H2O (6) were obtained in the crystalline form. Their crystal structures reveal the formation of hydrogen-bonded networks (chains and layers) built from hypodiphosphate anions and acid molecules, assisted by water molecules in the hydrates. The networks are embedded in the tetraalkylammonium cations' framework. Free acid molecules are present in all of the studied substances. Compound 5 was formed during a solid state dehydration reaction of 4, which has taken place as single-crystal-to-single-crystal transformation. Water removal is associated with a symmetry increase and structure reconstruction, with the change in the conformation of one of the hypodiphosphate anions, from the usual staggered into a rarely occurring eclipsed conformation. In 1, 3, 4 and 5 the formation of clusters with antielectrostatic hydrogen bonding (AEHB) was observed.


 Please wait while we load your content...
Please wait while we load your content...