SmFeO3 and Bi-doped SmFeO3 perovskites as an alternative class of electrodes in lithium-ion batteries
Abstract
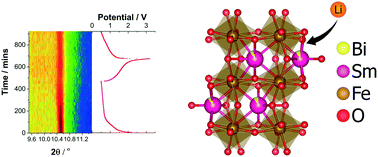
The search for new electrodes in alkali-ion batteries requires the investigation of a variety of classes of materials, each showing subtly different crystal structure motifs or frameworks. The structure–electrochemistry of SmFeO3 and Sm0.92(2)Bi0.08(2)FeO3 electrode materials are characterized by in situ and ex situ X-ray diffraction (XRD), galvanostatic charge/discharge cycling and electrochemical impedance spectroscopy. SmFeO3 and Sm0.92(2)Bi0.08(2)FeO3 electrodes deliver first discharge capacities of 450 and 550 mAh g−1, and their corresponding reversible discharge capacities are 300 and 400 mAh g−1, respectively, following this cycle at a current rate of 5 mA g−1. Interestingly, after the 20th cycle, SmFeO3 retains 98% of its capacity from previous cycles whereas Bi-doped SmFeO3 appears to lose its capacity extremely fast. In situ synchrotron XRD data suggests that discharge results in a partial loss of crystallinity, while charging slightly recovers the crystallinity of SmFeO3 but not of Bi-doped SmFeO3, which is also confirmed by ex situ XRD data. The lack of crystallinity recovery could be related to the poor higher rate performance observed for this doping regime. This work shows the potential of this class of materials as electrode materials, unconventional but possibly reliable.


 Please wait while we load your content...
Please wait while we load your content...