Probing hydrogen and halogen-oxo interactions in uranyl coordination polymers: a combined crystallographic and computational study†
Abstract
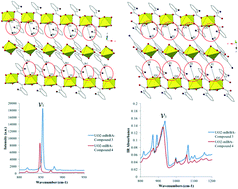
The syntheses and crystal structures of four compounds containing the UO22+ cation and either benzoic acid (1), m-chlorobenzoic acid (2), m-bromobenzoic acid (3), or m-iodobenzoic acid (4) are described and the vibrational spectroscopic properties for compounds 3 and 4 are reported. Single crystal X-ray diffraction analysis of these materials shows that uranyl oxo atoms are engaged in non-covalent assembly via either hydrogen (1 and 2) or halogen bonding (3 and 4) interactions. The halogen bonding in compounds 3 and 4 is notable as the crystallographic metric percentage of the sum of the van der Waals radii indicates these interactions are of similar strength. Characteristics of the halogen-oxo interactions of 3 and 4 were probed via Raman and infrared spectroscopy, which revealed significant differences in stretching frequency values for the two compounds. Additionally, compounds 3 and 4 were characterized via quantum chemical calculations and density-based quantum theory of atoms in molecules (QTAIM) analysis, which indicated that the I-oxo interaction in 4 is likely the stronger of the two interactions, with differences between the two interactions resulting from both inductive effects and halogen polarizability.


 Please wait while we load your content...
Please wait while we load your content...
