Polyamorphism and frustrated crystallization in the acid–base reaction of magnesium potassium phosphate cements†
Abstract
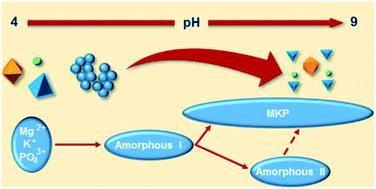
Magnesium potassium phosphate cements are a class of acid–base cements for bioengineering and civil engineering applications. The kinetics of their chemical reaction was investigated in situ with isothermal conduction calorimetry and powder X-ray diffraction, quantifying amorphous and crystalline products. The first reaction step, dissolution of MgO, with an apparent activation energy of 71 kJ mol−1, dictates the time evolution of two amorphous intermediate precursors and the crystalline product. The early crystallization of the latter has been described with an Avrami equation with an apparent activation energy of 81 kJ mol−1, pointing to a mechanism of deceleratory nucleation and growth in one direction, compatible with the acicular crystal habit observed with electron microscopy. The observed polyamorph transformation is controlled by a complex interplay between kinetic and thermodynamic factors, in which the changes in the chemical environment (increase in pH) driven by the MgO dissolution, play a crucial role. It is proposed that the onset of the amorphous–amorphous transformation hinders crystallization by decreasing ion mobility, raising the energy barriers to structural reorganization. The rate of MgO dissolution depends on the reactivity of the powder and the parameters of the mix (such as the amount of liquid) and influences the reaction pathways, impacting on material performance.


 Please wait while we load your content...
Please wait while we load your content...