Two lanthanide metal–organic frameworks constructed from tris(4-carboxyphenyl)phosphane oxide with gas adsorption and magnetic properties†
Abstract
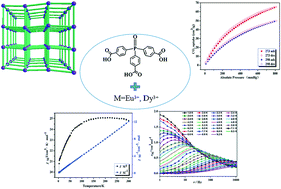
Two lanthanide metal–organic frameworks (LOFs), namely, {[Eu(L3−)(H2O)]·5H2O}n (1) and {[Dy(L3−) (H2O)]·2CH3CN}n (2), have been synthesized based on tris(4-carboxyphenyl)phosphane oxide (H3L) and further characterized by powder X-ray diffraction (PXRD), IR spectroscopy, elemental analyses and thermogravimetric (TG) analyses. X-ray diffraction analyses showed that complexes 1 and 2 are isostructural, and thus the structure and gas adsorption properties of complex 1 and the magnetic properties of 2 were described as a representative in detail. Complex 1 appears to have a 3D 6-connected {412·63} net based on binuclear {Eu2(COO)2} SBUs. Furthermore, the adsorption properties of 1 have been investigated using N2, H2, CO2 and CH4 gases. 1 has high CO2 uptake and exhibits highly selective adsorption of CO2 over CH4 at room temperature, which allows 1 to have a potential application in gas separation and purification. Finally, its full magnetic properties revealed that complex 2 displays antiferromagnetic coupling between DyIII ions and single-molecule magnet (SMM) behavior. According to the Arrhenius law, the anisotropy barrier (Ueff) and pre-exponential factor (τ0) were extracted by fitting of the alternating current data, giving the values of 53.5 K and 3.1 × 10−7 s, respectively. The afforded α parameter is less than 0.10, suggesting a single relaxation process.


 Please wait while we load your content...
Please wait while we load your content...