Tuning charge-assisted and weak hydrogen bonds in molecular complexes of the proton sponge DMAN by acid co-former substitution†
Abstract
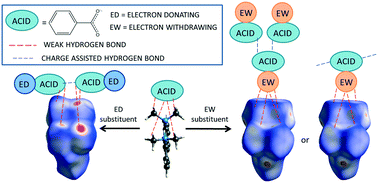
Nine new molecular complexes of the proton sponge 1,8-bis(dimethylamino)naphthalene (DMAN) with substituted benzoic acid co-formers have been engineered with varying component stoichiometries (1 : 1, 1 : 2 or 1 : 3). These complexes are all ionic in nature, following proton transfer between the acid co-former and DMAN; the extracted proton is held by DMAN in all instances in an intramolecular [N–H⋯N]+ hydrogen bond. A number of structural features are common to all complexes and are found to be tunable in a predictable way using systematic acid co-former substitution. These features include charge-assisted hydrogen bonds formed between acid co-formers in hydrogen bonding motifs consistent with complex stoichiometry, and weak hydrogen bonds which facilitate the crystal packing of DMAN and acid co-former components into a regular motif. Possible crystal structure tuning by co-former substitution can aid the rational design of such materials, offering the potential to target solid-state properties that may be influenced by these interactions.


 Please wait while we load your content...
Please wait while we load your content...