Preparation, crystal structure and solution-mediated phase transformation of a novel solid-state form of CL-20†
Abstract
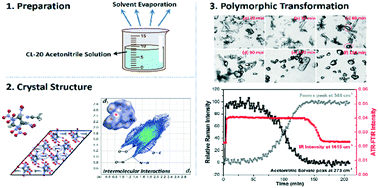
Herein, the 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane (CL-20) acetonitrile solvate, a novel solid-state form of CL-20, was first discovered and characterized by various analytical techniques. The results of single-crystal X-ray diffraction (SCXRD) indicate that it is a channel-type solvate. Intermolecular interactions inside the crystal were investigated to elucidate the assembly behaviors with the assistance of the Hirshfeld surface analysis. The solvation profile and solubility data were determined to identify the thermodynamic stability of the acetonitrile solvate and form ε. The solution-mediated phase transformation (SMPT) from the acetonitrile solvate to form ε in acetonitrile–chloroform mixed solvents was online monitored by attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy and in situ Raman spectroscopy. The results reveal that the nucleation and growth of the form ε is the rate-determining step. Moreover, the Johnson–Mehl–Avrami (JMA) model was employed to obtain deeper insights into the transformation kinetics. Furthermore, the effects of solvent composition, solid loading, and agitation rate were examined. This study provides an alternative approach for obtaining the desired form ε crystals and lays a foundation for the optimization of the crystallization process in the future.


 Please wait while we load your content...
Please wait while we load your content...