Understanding the role of oxygen ion (O2−) activity in 1-D crystal growth of rutile TiO2 in molten salts
Abstract
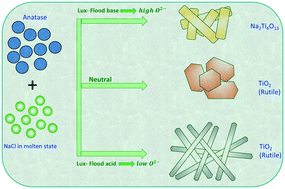
Controlled synthesis of nanostructured materials using facile and easily scalable synthesis techniques is highly attractive for large-scale production of nanomaterials. In this regard, molten salt synthesis is a well-established technique for large-scale production of nanostructured materials. Few reports have demonstrated the applicability of the molten salt technique for high-aspect-ratio one-dimensional rutile TiO2 synthesis. However, the crystal growth mechanism of 1-D TiO2 in the molten salt is not well understood. Here, various sets of experiments have been delivered to investigate 1-D rutile TiO2 crystal growth starting from anatase TiO2 precursors in molten NaCl with the presence of various inorganic oxy-additives. It was found that the oxygen ion (O2−) activity of the molten salt matrix, which can be controlled by oxy-additives, is the decisive factor for the formation of the 1-D structure. The (NaPO3)6 additive, which reduces the O2− activity of the molten salt matrix, increased the solubility of anatase TiO2. The increased solubility facilitates the easy mobility of ions to the growth site where the crystallographic surface energy is high. The natural tendency to minimize the total surface energy causes the crystal to grow in a particular direction, which eventually leads to 1-D rutile TiO2 nanoparticles.


 Please wait while we load your content...
Please wait while we load your content...