Crystal structures and phase transformation of two novel solvates of valnemulin hydrochloride†
Abstract
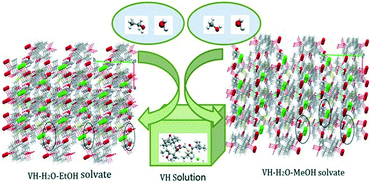
Two novel solvates of valnemulin hydrochloride (VH), one is valnemulin hydrochloride water–ethanol solvate (VHWES) and the other is valnemulin hydrochloride water–methanol solvate (VHWMS), were first crystallized successfully. The structural and spectral characteristics of VHWES and VHWMS were studied by single crystal X-ray diffraction (SCXRD), Fourier transform infrared spectroscopy (FT-IR) and Raman spectroscopy. It was found that both solvates crystallized in the monoclinic system with a P2 space group, and comprised protonated ammonium moieties, hydrochloride anions, water molecules and solvent molecules (ethanol or methanol) which are held together by a number of hydrogen bonds. The thermal characterization of VHWES and VHWMS was conducted through DSC/TG analysis. The results indicated that both of them would undergo a solvent loss over a certain temperature range, and then return to their original amorphous form. The solution-mediated phase transformation from VH to VHWES was in situ investigated by using PVM and Raman spectroscopy, and the kinetic parameters of the transformation process were also determined. It was found that the nucleation of VHWES was the limiting step of the transformation process, and the transformation rate accelerated with increasing temperature.


 Please wait while we load your content...
Please wait while we load your content...