Ternary Pt–Pd–Ag alloy nanoflowers for oxygen reduction reaction electrocatalysis†
Abstract
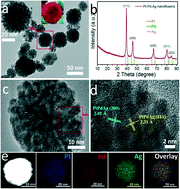
Elemental composition, dimensionality and morphology are the main factors that influence the catalytic activity and stability of platinum-based and noble metal alloy nanocatalysts. Herein, we present a facile and cost-efficient approach to synthesize ternary alloy Pt–Pd–Ag nanoflowers by co-reduction of the metal precursors in aqueous solutions in a suitable molar ratio, involving hexamethylenetetramine as a structure-directing agent and ascorbic acid as a reducing agent. Both the presence of the aforementioned agents and the interaction between the component metals allow good control over the anisotropic growth of the ternary alloy nanostructure. The as-prepared nanocomposite demonstrates superior electrocatalytic activity towards the oxygen reduction reaction in acidic medium, compared with commercial Pt/C (20 wt%) and Pd/C (10 wt%) catalysts, as well as sufficiently good stability, as demonstrated by the accelerated durability test. The electrocatalytic activity of irregularly shaped nanoparticles of the same elemental composition was also measured, which revealed that the nanoflowers were superior catalysts owing to their specific morphological characteristics. Moreover, the nanoflowers displayed a relatively low electrochemical surface area decay (from 100% to 80.82%) due to their three-dimensional open-framework surface which can limit the aggregation of individual particles.


 Please wait while we load your content...
Please wait while we load your content...