Ab initio structure solution and thermal stability evaluation of a new Ca(ii) 3D coordination polymer using synchrotron powder X-ray diffraction data†
Abstract
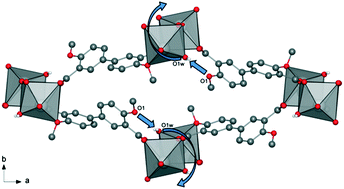
A new coordination polymer was synthesized hydrothermally using 4,4′-dimethoxy-3,3′-biphenyldicarboxylic acid (H2dmbpdc) and Ca(NO3)2·4H2O. The structure of the new compound, Ca(dmbpdc)(H2O)2 (CPO-70-Ca), was determined from powder X-ray diffraction (PXRD) data using simulated annealing, followed by Rietveld refinement of the structure. The framework is based on one-dimensional metal carboxylate chains that are linked by the organic linker into a three-dimensional network with rhombic channels. The as-synthesized material resembles our recently reported compound CPO-69-Ca, and shares the same sra topology. The asymmetric unit comprises one calcium atom, one fully deprotonated linker, one water molecule coordinated to the metal cation as well as one solvent water molecule located in the rhombic channels. The metal cation is eight-coordinate, forming an irregular coordination polyhedron. The thermal behavior of CPO-70-Ca was investigated in an in situ structural analysis by variable temperature powder X-ray diffraction. This study revealed an interesting response to solvent removal from the as-synthesized structure. The results include a partially desolvated version of CPO-70-Ca and a structural transformation to CPO-69-Ca upon full desolvation.


 Please wait while we load your content...
Please wait while we load your content...