A series of transition metal coordination polymers based on a rigid bi-functional carboxylate–triazolate tecton†
Abstract
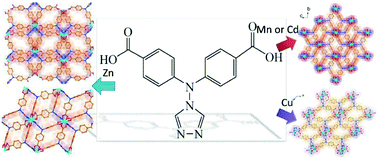
By utilizing the pre-designed bi-functional ligand 4-{bis(4-benzoic)amino}-4H-1,2,4-triazole (H2L), five new transition-metal-based coordination polymers, namely, {[Zn(L)]·H2O·DMA}n (1), {[Zn2(L)2]·DMF}n (2),{[Mn(L)]·DMF}n (3), {[Cd(L)]·DMA}n (4) and [Cu3(OH)2(L)2]n (5) have been constructed and structurally characterized. In 1, the tetrahedral zinc ion was incorporated in the final structure. However, complexes 2, 3 and 4 bear 1D Zn-, Mn- and Cd-chains composed of metal ions, triazole and carboxyl groups, respectively. Differently, 1D [Cu4(μ3-OH)2] chains had been stabilized in the 3D structure of 5. In addition, the luminescence or magnetic properties of 1–5 had been correspondingly investigated with the consideration of their crystal structure. The whole research results manifest that utilizing a bi-functional ligand containing carboxyl and triazole groups is an efficient building method in constructing functional CPs.


 Please wait while we load your content...
Please wait while we load your content...