Organic NLO material with H-bonded 1D helical self-assembly: synthesis, X-ray crystal structure, DFT calculations, SHG measurements and thermal studies of (5Z,6E)-1,10-phenanthroline-5,6-dione dioxime†
Abstract
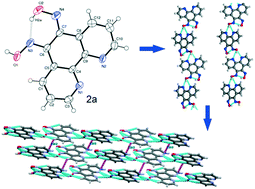
(5E,6Z)-1,10-Phenanthroline-5,6-dione dioxime (2a), an organic nonlinear optical (ONLO) material, is synthesized and characterized by X-ray diffraction: a = 4.8736(5) Å, b = 8.5549(10) Å, c = 12.5992(16) Å, α = 90°, β = 91.428(4)°, γ = 90°, V = 525.14(11) Å3, z = 2 and monoclinic, Pc. The crystal structure of 2a reveals an infinite 1D helical assembly formed by intra- and intermolecular hydrogen bonding retaining the microscopic asymmetry of the molecule leading to its enhanced NLO behaviour. The second harmonic generation (SHG) efficiency of 2a is 0.83 times as great as that of urea, measured by the Kurtz and Perry powder method. The crystal structure, UV-Vis-NIR spectrum, TD-DFT calculations, SHG measurements and thermal studies indicate that the structural, electronic, optical, and thermal properties make this molecule exhibit second order NLO characteristics for optical applications. The synthesis, characterization and SHG activity of 1 and 3 are also reported.


 Please wait while we load your content...
Please wait while we load your content...