Pore wall fluorescence labeling of covalent organic frameworks†
Abstract
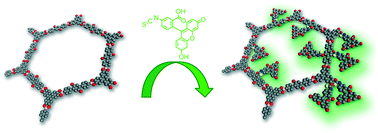
A novel covalent organic framework (COF) based on terphenyldiboronic acid exhibiting open pores of about 4.1 nm is presented. The newly synthesized COF features accessible hydroxyl groups at the inner and outer pore walls. These functional groups serve as anchor sites for a fluorescence label installed through a post-synthetic modification approach. By forming o-thiocarbamate bonds, fluorescein molecules were immobilized on the inner and outer surfaces of the pore system, respectively. This grafting reaction was also extended to a second COF (COF-5) and to other grafted moieties.
- This article is part of the themed collection: Covalent organic frameworks and organic cage structures


 Please wait while we load your content...
Please wait while we load your content...