Experimental evidence of a 3-centre, 2-electron covalent bond character of the central O–H–O fragment on the Zundel cation in crystals of Zundel nitranilate tetrahydrate†
Abstract
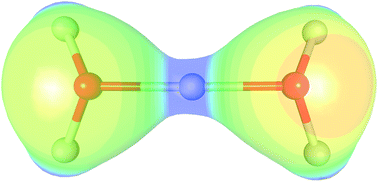
The charge density of the Zundel cation in the crystal of 2[H5O2]+[C6O8N2]2−·4H2O is studied by X-ray diffraction and periodic DFT calculations. The covalent nature of the central O–H–O bonds in the Zundel cation is revealed: the electron density at the two O–H critical points is about 1.0 e Å−3, corresponding to a three-centre, two-electron system. The bond orders derived from the topology of the electron density are 0.27–0.41, which are more similar to those of single O–H bonds (bond order 0.45–0.51) than those of H⋯O hydrogen bonds (bond orders = 0.05–0.10).


 Please wait while we load your content...
Please wait while we load your content...