Four new zinc(ii) diphosphonates obtained via an ionothermal route: crystal structures and phase transformation behaviour†
Abstract
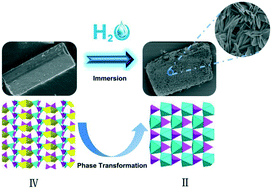
Four new crystalline zinc diphosphonates, Zn2(xbp) (I), Zn2(H2O)2(xbp) (II), Na2Zn3(H2O)2(xbp)2 (III) and NaZn(H2xbp)0.5(xbp)0.5·1.5H2O (IV) were ionothermally synthesized from tetraethyl-p-xylylenebisphosphonate (Texbp) and Zn(OAc)2 in a deep eutectic solvent of oxalic acid and tetramethylammonium chloride. Their structures were determined by single-crystal X-ray diffraction and had a similar characteristic of inorganic layers pillared by organic parts of ligands on the whole. The introduction of sodium ions leads to two Na-containing zinc diphosphonates (III and IV), wherein the sodium ions adopt NaO4 tetrahedra or NaO5 square bipyramids in the inorganic layers. Interestingly, compound IV could completely convert to sodium-free compound II after soaking in water, following a process from the disintegration of the single crystal to in situ growth of another crystal. The phase transformation process was illustrated by using powder XRD, SEM, and ICP elemental analysis technologies. This work also demonstrates the structural diversity in an ionothermal reaction and the potential for preparing novel solids that are sensitive to hydrolysis in the presence of excess water.


 Please wait while we load your content...
Please wait while we load your content...